Office of Risk Management and Compliance
Procurement
and Inventory Management of
Hazardous Materials in
Laboratories

The NUS Laboratory
Materials Management System
(LMMS) was developed in 2016
to enhance the management of
regulated chemicals.
Introduced to
laboratory-based departments
in phases, the campus-wide
implementation of the LMMS
was completed in October
2021, with the introduction
of additional modules for
the management of
radioactive and biological
materials. All regulated
hazardous materials shall be
inventorised in LMMS.
The LMMS serves as an
electronic inventory
management tool that
| 1.
|
Provides
a real-time overview
of materials (i.e.
chemicals, radioactive
and biological) that
are in possession by
the respective
laboratories. |
| 2. |
Ensures that
regulated hazardous
material stocks are
within licensed
quantities and/ or are
registered with the
Safety & Health
Management, ORMC (i.e.
regulated biological
materials). |
| 3. |
Is mobile-enabled
and will allow
authorised personnel
to have quick access
to inventory records
in emergency response
situations. |
To provide a seamless
integration of procurement
with the inventory
management system, the Laboratory
Materials Purchase
Requisition System
(LMPRS) was introduced in
2019 with a chemical module
and was later expanded to
include 2 additional modules
for the purchase of
radioactive and biological
materials. All materials
purchased through LMPRS are
directly inventorised into
the Principal Investigator’s
account in LMMS.
With reference to Directive
1902: Requirements
on Procurement and
Inventory of Chemicals,
and Procurement
Circular CPO/2022/002,
all hazardous materials
shall be procured using
LMPRS.
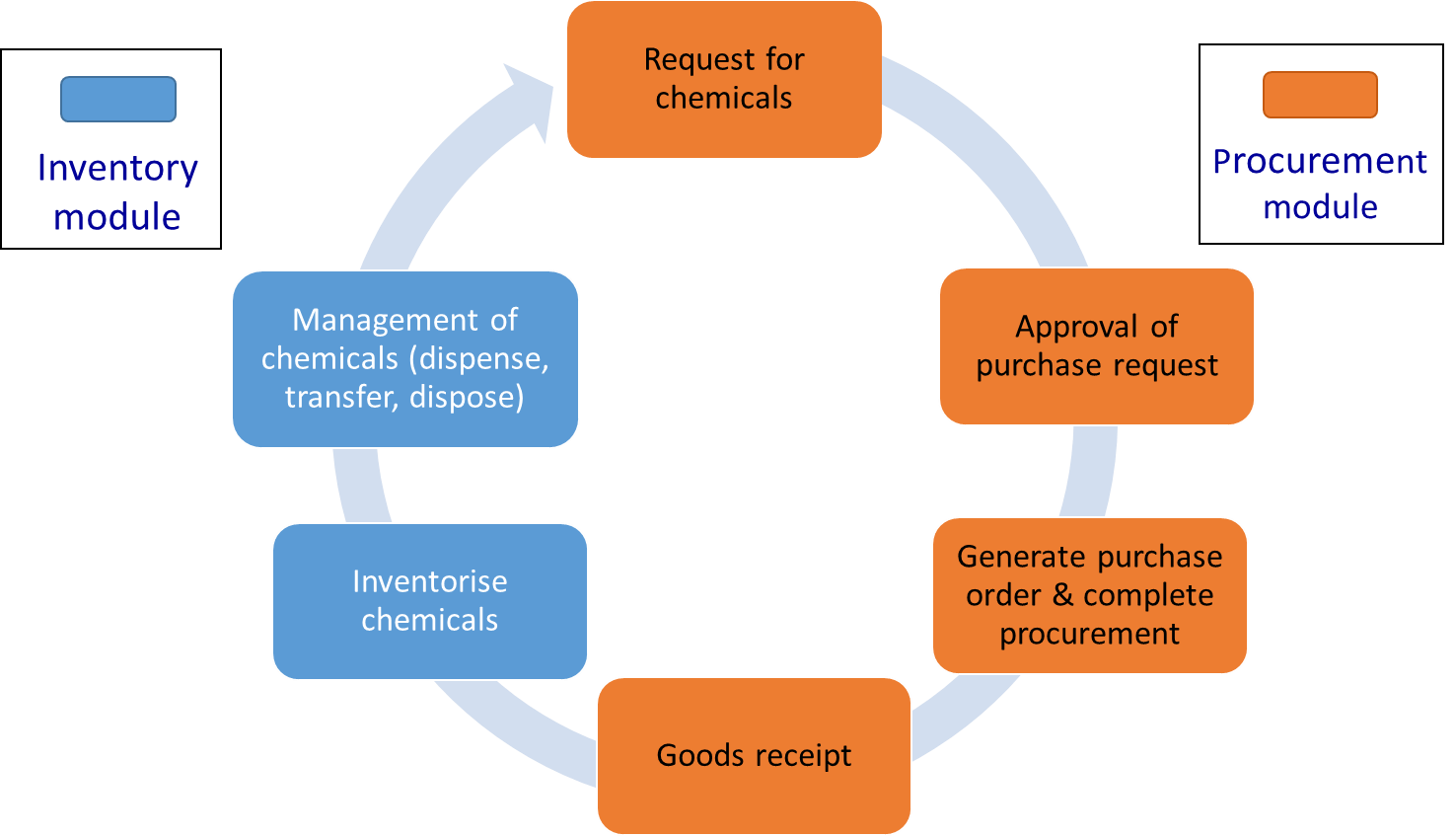
Diagram:
Overview of chemical
procurement and inventory
management process in LMMS and
LMPRS
User Guides
FAQs
| LMMS
Chemical Inventory
Module FAQ |
FAQ
FAQ covers topics such
as LMMS User Access,
inventory management
functions, barcode
scanners and labels |
LMMS
Biological Inventory
Module FAQ |
FAQ
FAQ covers topics
related to
inventorisation of
biological materials |
| LMMS
Radioactive Materials
Inventory Module FAQ |
FAQ
FAQ covers topics
related to
inventorisation of
radioactive materials |
Exempted Items
The use of LMPRS is exempted for
the purchases of the following
items. These items can still be
purchased via LMPRS but it is
not mandatory to do so.
It is the responsibility of the
requestor/ approver to evaluate
and ensure that the materials
(1) are not regulated or (2) do
not contain any regulated
components.
| S/N |
Products |
Examples |
| 1 |
Items
relating to molecular
biology/ genetic
modification/ general
life sciences |
a.
Oligonucleotides,
primers, restriction
enzymes
b. Antibodies
(monoclonal/polyclonal/recombinant)
c. Culture media, Buffer
solutions
d. Aassay kits
e. Dyes |
| 2 |
Materials
used for filter and
chromatographic
purification |
a.
Silica gel, zeolite and
molecular sieves |
| 3 |
Liquid
nitrogen |
|
| 4 |
Dry
ice |
|
| 5 |
Over-the-counter
products that are not
controlled by regulators |
a.
Lubricant
b. Paints
c. Hand sanitisers,
surface disinfectants
d. Over-the-counter
medication and health
supplements |
Testimonial
"This is Wen from
the Department of
Pharmacy and I have
started using LMMS
recently as a new PI.
I would like to express
my sincere thanks to you
and your colleagues for
delivering and managing
such an excellent
management system. LMMS
at NUS is the dream
system to manage
laboratory materials
(e.g. chemicals),
allowing PI to have
instant access to
important information
such as the quantity of
a specific chemical in a
specific location, which
can be critical in a
safety-relevant
situation. I look
forward to the continual
evolution of LMMS to
help PIs to manage their
laboratories more
safely, sustainably and
efficiently.”
|

|
Dr
Chen Wenqian,
Assistant Professor
Department
of Pharmacy
Faculty
of Science
|
Support
Please contact the
following personnel
pertaining to the use of
LMMS and LMPRS:
| Department |
Type
of Enquiries |
Contact
Person |
| Safety
and Health Management,
ORMC |
1.
Enquiries on LMMS:
a. Data Configuration |
Chemical Module
Ms Clarissa Wong
Senior Executive
Email: clarissawong@nus.edu.sg
Telephone: 6601 5048
Mr. Joel Swee Dao Wen
Associate Director
Email: joelswee@nus.edu.sg
Telephone: 6516 5725
Radioactive Module
Dr Andrew Ng
Manager
Email: andrewng@nus.edu.sg
Telephone: 6516 7427
Biologial Materials
Module:
Dr Lawrence Sie Eng Kean
Assistant Senior Safety & Health Manager
Email: lawrencesie@nus.edu.sg
Telephone: 6516 1051
|
| Central
Procurement Office (CPO) |
1.
Enquiries on LMPRS
2. Enquiries on
procurement procedures |
Michael Ng
Senior Manager (Business
Analyst)
Email: ngjk@nus.edu.sg
Telephone: 6601 5525
|

|
|