|
1
|
INTRODUCTION
|
|
The National
University of
Singapore has
been granted
an
institutional
licence to
possess Risk
Group 2
veterinary
biologics on 1
April 2013 by
Agri-Food
&
Veterinary
Authority
(AVA) of
Singapore,
under the
Animals and
Birds Act
(Chapter 7).
|
|
With effect
from 1 April
2019, the
Agri-Food
&
Veterinary
Authority of
Singapore
(AVA) has been
restructured
to form
Singapore Food
Agency (SFA)
and the Animal
&
Veterinary
Service (AVS)
under National
Parks Board
(NParks), and
the NUS
institutional
Licence to
Possess
Veterinary
Biologics is
regulated
under
NParks/AVS.
|
|
The NUS
institutional
licence covers
Risk Group 2
veterinary
biologics in
the
Select List
on AVS
website, and
includes
organisms /
registered
vaccines /
proteins /
peptides /
other genetic
materials of
Risk Group 2
veterinary
biologics.
|
|
|
|
2.
|
REGISTRATION
TO POSSESS
RISK GROUP 2
VETERINARY
BIOLOGICS
|
|
|
|
AVA
Licence
held by
Principal
Investigators
|
|
The AVA
Licence to
Possess
Veterinary
Biologics
(LPVB) held by
individual
Principal
Investigators
for their Risk
Group 2
veterinary
biologics will
no longer be
valid. These
Principal
Investigators
must now
register their
possession of
the Risk Group
2 veterinary
biologic with
the Office of
Safety, Health
and
Environment in
accordance
with the terms
and conditions
of this NUS
institutional
licence.
|
|
|
|
New
Registration
|
|
Any Principal
Investigator
in NUS
(located in
Kent Ridge
campus) who
intends to
import /
acquire a
biological
agent should
refer to the
Select List
to determine
whether the
biological
agent is
regulated by
NParks/AVS. If
it is a Risk
Group 2
veterinary
biologic
regulated by
NParks/AVS,
Principal
Investigators
must submit
the
Registration
via EHS360
Institutional
Approval
module
(please select
LPVB).
|
|
|
|
If the
infectious
agent is not
on the Select
List but has
the potential
for zoonotic
transmission
or if it is a
Risk Group 3
veterinary
biologic, then
the Principal
Investigator
must complete
and submit an
NParks form
"Evaluation
of Risk
Posed by the
Importation
of Animal
Pathogens
into
Singapore"
to OSHE for
further
evaluation by
NParks/AVS.
Please consult
OSHE staff if
in doubt.
|
|
|
|
Submission
of
Documents
|
|
Principal
Investigators
must submit
the following
documents on
EHS360
Institutional
Approval
module
:
|
|
|
a.
|
Experiment-based
Risk
Assessments
|
|
b.
|
Standard
Operating
Procedures
|
|
|
|
|
The
experiment-based
Risk
Assessments
and Standard
Operating
Procedures
shall include
the following:
|
|

|
Importation/exportation
|

|
Receipt
and
handling
of
incoming
regulated
biologics
including
incident
reporting
if
there
is
leakage
detected
in
packaging
received
|

|
Transportation
|

|
Access
control
(granting
access
to lab
and
veterinary
biologics
in
storage)
|

|
Experimental
work
|

|
Biological
spills
|

|
Decontamination
and
disposal
of
biohazardous
waste,
including
routine
disinfection
of
potentially
contaminated
surfaces
|

|
Waste
disposal
|

|
Emergency
response
procedures,
including
spill
inside/outside
BSC,
accidental
exposure,
fire,
loss
of
electrical
power
|
|
|
|
|
3
|
GUIDELINES
ON
REGISTRATION
&
IMPORT/TRANSFER
|
|
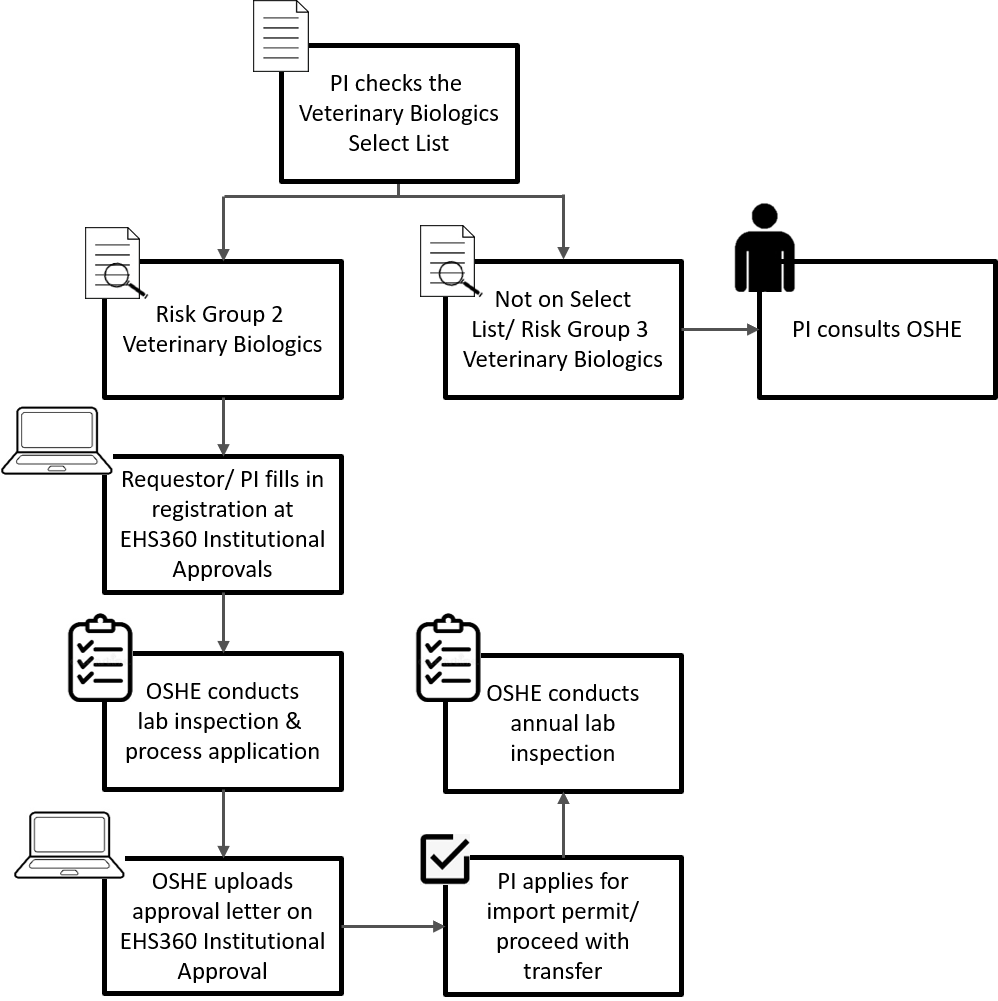
The procedures
for
registration
to possess
Risk Group 2
veterinary
biologics, and
import/transfer
is outlined in
the
NUS
Laboratory
Biorisk
Management
Manual
.
|
|
|
|
A summary flow
chart on the
process is
outlined
below:
|
|
|
|
|
|
|
|
4.
|
CONTACT
PERSON
|
|
For submission
of forms and
enquiries,
please contact
Dr Lawrence
Sie at
lawrencesie@nus.edu.sg
/ 65161051 or
Dr Rajkumar
Ramamoorthy at
rajkumar@nus.edu.sg
/ 66011169.
|