| 1. |
INTRODUCTION
|
| |
The
Global
Polio
Eradication
Initiative
(GPEI) led by
World Health
Organization
(WHO) was
founded in
1988 with the
aim to prevent
incidence of
paralytic
poliomyelitis
through the
complete
eradication
and
containment of
polioviruses1.
More
information on
The WHO’s
global action
plan for
poliovirus
containment is
provided in
the document,
WHO Global
Action Plan
for poliovirus
containment (GAPIII).2 |
| |
In
March 2015,
Ministry of
Health (MOH)
conducted a
national
survey to
establish the
national
inventory of
poliovirus
types 1, 2 and
3 infectious
materials
(IMs), in
preparation
for the
containment of
poliovirus
materials (MOH
Ref. No.: MH
78:70, letter
dated 14 Mar
2015 entitled
Surveillance
of Poliovirus
Inventory). |
| |
In
October 2018,
MOH conducted
a national
survey on poliovirus
potentially
infectious
materials
(PIMs) as
such materials
also pose a
risk of
accidental
poliovirus
reintroduction
into the
community e.g.
from the
laboratory, in
the
post-eradication
era. (MOH Ref.
No.: MH 34:18,
letter dated
29 Oct 2018
entitled
Survey to
Update the
National
Inventory of
Poliovirus
Materials). |
| |
A
Guidance
to Minimize
Risks for
Facilities
Collecting,
Handling or
Storing
Materials
Potentially
Infectious for
Polioviruses3
was published
by WHO in 2018
to provide
guidance on
collecting,
handling or
storing of
PIMs. |
|
In January
2019, MOH
conducted an
Information
session on
poliovirus
potentially
infectious
materials4
and MOH
required each
facility that
has poliovirus
IMs and/or
PIMs to submit
a completed Facility
Reporting Form
1 to MOH,
and facilities
retaining oral
poliovirus
vaccine
(OPV)/Sabin
type 2 PIMs
are required
to submit a
Risk
Mitigation
Plan to MOH. |
|
|
| 2. |
DEFINITION
OF POLIOVIRUS
INFECTIOUS
MATERIALS (PV
IMs) AND
POLIOVIRUS
POTENTIALLY
INFECTIOUS
MATERIALS (PV
PIMs) |
|
The
definition of
poliovirus IMs
or poliovirus
PIMs is
available in
MOH’s
presentation
slides, Information
Session on
Poliovirus
Potentially
Infectious
Materials.4
In summary: |
| |
b. Poliovirus
potentially
infectious
materials (PV
PIMs) are
defined as
faecal,
respiratory,
concentrated
sewage samples
or derivatives
of such
samples,
collected at a
time of
poliovirus
circulation in
the community,
or when live,
attenuated
poliovirus
vaccine is
used;
regardless of
the purpose of
sample
collection.
-
i. Wild
poliovirus
(WPV) PIMs:
Any of the
above sample
types or
derivatives of
such samples,
collected at a
time of wild
poliovirus
circulation in
the community.
-
ii. Vaccine-derived
poliovirus
(VDPV) PIMs:
Any of the
above sample
types or
derivatives of
such samples,
collected at a
time of
vaccine
derived
poliovirus
outbreak in
the community.
-
iii. Oral
poliovirus
vaccine
(OPV)/Sabin
PIMs: Any
of the PV PIM
sample types
or derivatives
of such
samples,
collected at a
time when
live,
attenuated
poliovirus
vaccine is
used in the
community.
|
| |
|
| 3. |
POLIOVIRUS
INFECTIOUS
MATERIALS (PV
IMs), WILD
POLIOVIRUS
POTENTIALLY
INFECTIOUS
MATERIALS (WPV
PIMs) OR
VACCINE-DERIVED
POLIOVIRUS
POTENTIALLY
INFECTIOUS
MATERIALS
(VDPV PIMs)
|
| |
Facilities
in possession
of or
intending to
acquire/obtain
poliovirus
IMs, WPV PIMs
or VDPV PIMs
are required
to contact
OSHE for
advice, as
these
facilities
need to be
certified as
poliovirus-essential
facility
(PEF). |
| |
|
| 4. |
ORAL
POLIOVIRUS
VACCINE (OPV)
/ SABIN
POTENTIALLY
INFECTIOUS
MATERIALS
(PIMs) |
|
People who
receive OPV
may shed the
virus and can
infect others,
especially
those who are
not
vaccinated. In
areas with low
vaccination
rates, the OPV
virus can
continue to
infect new
individuals.
In rare cases,
the OPV virus
can accumulate
changes over
time and
become like
WPV. These new
viruses are
called VDPV
and can cause
polio disease. |
|
Facilities
that collect,
handle and
store clinical
and
environmental
samples
present a
poliovirus
transmission
risk if the
samples were
collected
where WPV or
VDPV was
circulating,
or OPV was
being used. |
|
Any
faecal,
respiratory
secretion or
concentrated
sewage samples
collected in
the community
and stored by
a facility are
considered as
Poliovirus
Potentially
Infectious
Materials (PV
PIMs), which
include:
• faecal or
respiratory
secretion
samples and
their
derivatives
(e.g. stool
suspensions,
extracted
nucleic acids,
etc.)
collected for
any purpose in
a geographic
area where
wild
poliovirus
(WPV)/circulating
vaccine-derived
poliovirus
(cVDPV) is
present or OPV
is being used
at the time of
collection;
• products
of such
materials
(above) from
PV-permissive
cells or
experimentally
infected
polio-susceptible
animals;
•
uncharacterized
enterovirus-like
cell culture
isolates
derived from
human
specimens from
countries
known or
suspected to
have
circulating
WPV/VDPV or
use of OPV at
the time of
collection;
•
respiratory
and enteric
virus stocks
derived from
PV PIM and
handled under
conditions
conducive to
maintaining
the viability
or enabling
the
replication of
incidental PV;
and
•
environmental
samples (i.e.
concentrated
sewage, waste
water)
collected from
areas known or
suspected to
have
circulating
WPV/VDPV or
use of OPV at
the time of
collection |
| |
Respiratory
tract samples
include saliva
and oral swab
which are
defined under
“nasopharyngeal,
oropharyngeal
and other
upper
respiratory
tract
secretions”
mentioned on
page 10 of the
WHO guidance
document.3 |
|
In addition
to sample
type,
determining
whether
samples are
WPV2/VDPV2 or
OPV/Sabin2 IM
or PIM is
based on collection
period and
country of
origin.
Refer to Annex
2 of WHO
guidance
document3
for country
and
territory-specific
poliovirus
data. |
|
The flow
chart below
provides a
summary on the
process to
determine PV
PIMs. |
| |
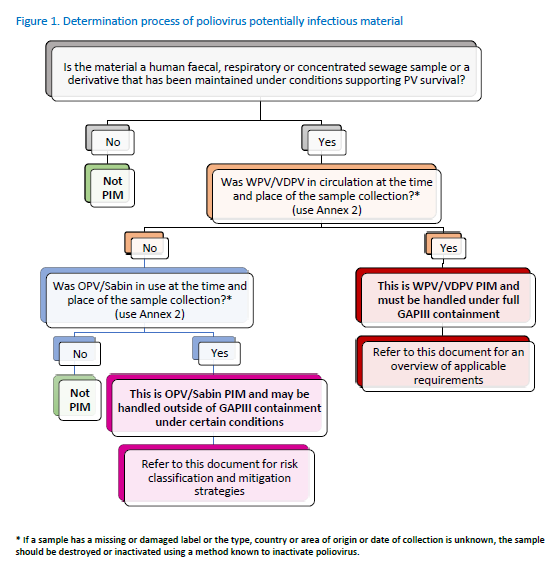
|
| |
Source:
WHO’s Guidance
to Minimize
Risks for
Facilities
Collecting,
Handling or
Storing
Materials
Potentially
Infectious for
Polioviruses4 |
|
Please refer
to WHO’s Guidance
to Minimize
Risks for
Facilities
Collecting,
Handling or
Storing
Materials
Potentially
Infectious for
Polioviruses4
for detailed
information
for guidance
on OPV/Sabin
PIM samples. |
|
|
| 5. |
REGISTRATION
AND
DE-REGISTRATION
OF PV PIMs |
|
MOH requires
each new
facility
retaining PV
PIM to
complete and
submit a copy
of the Facility
Reporting Form
1 to MOH
once
collection of
PV PIM samples
begin. Please
submit the
completed Facility
Reporting Form
1 to OSHE
and OSHE will
facilitate the
submission to
MOH on behalf
of the
facility. |
|
Submission
of the
reporting form
is not
required for a
facility that
collects PV
PIM that will
be disposed
upon
collection and
immediate use,
i.e. no
retention of
PV PIM or its
derivatives. |
|
The Facility
Reporting Form
1 is
available here. |
|
Declaration
to MOH is
required for
destruction of
specific PV
PIM type or
all existing
PV PIM
inventory.
Please submit
either MOH
Form A or Form
B to OSHE and
OSHE will
facilitate the
submission to
MOH on behalf
of the
facility.
|
|
|
- • MOH
Form A: to
notify MOH of
destruction of
all existing
PV PIM
inventory
(complete),
with no
intention for
collection,
handling
and/or storage
of such
materials in
future;
|
|
- • MOH
Form B: to
notify MOH of
the
destruction of
specific
inventory type
(partial)
|
6.
|
NUS RISK
MITIGATION
PLAN FOR
OPV/SABIN TYPE
2 PIMs |
|
The NUS
Institutional
Biosafety
Committee has
released an NUS
Risk
Mitigation
Plan for Oral
Poliovirus
Vaccine/Sabin
Type 2
Potentially
Infectious
Materials
and it is
available here.
All NUS
laboratories/facilities
in possession
of and/or
handling
OPV2/Sabin2
PIMs shall
comply with
all the
relevant
requirements
in the NUS
Risk
Mitigation
Plan. |
|
|
| 7. |
POLIOVIRUS
POTENTIALLY
INFECTIOUS
MATERIALS
TRANSFER FORM |
|
MOH requires
facilities to
inform MOH of
OPV2/Sabin2
PIM transfer,
either between
two NUS
facilities or
from NUS to an
external
facility, as
well as to
communicate to
the recipient
to comply with
the relevant
risk
mitigation
measures. To
assist the
notification
of transfer to
MOH and
communication
of MOH’s
requirements
to the
recipient, the
NUS IBC has
released a Poliovirus
Potentially
Infectious
Materials
Transfer Form.
The NUS
transferor
shall complete
a Poliovirus
Potentially
Infectious
Materials
Transfer Form
and send a
copy of the
completed form
to the
recipient and
OSHE. OSHE
will then
assist to
notify MOH on
the transfer
of OPV2/Sabin2
PIM on behalf
of the
facility.
This form only
needs to be
filled in once
if the same
type of
samples is
transferred to
the same
recipient.
The Poliovirus
Potentially
Infectious
Materials
Transfer Form
is available here. |
|
|
| 8. |
CONTACT
PERSON |
|
For
enquiries on Facility
Reporting Form
1 or Poliovirus
Potentially
Infectious
Materials
Transfer Form,
please contact
Dr Suzette De
Leon at oshdlsn@nus.edu.sg
/ 66011703. |
| |
For
enquiries on NUS
Risk
Mitigation
Plan for Oral
Poliovirus
Vaccine/Sabin
Type 2
Potentially
Infectious
Materials,
please contact
Dr Lim Cheh
Peng at oshlimcp@nus.edu.sg
/ 65167088. |
|
|
9.
|
REFERENCES
|
|
[1] World
Health
Organization,
"Global Polio
Eradication
Initiative,"
[Online].
Available: http://polioeradication.org/. |
|
[2] World
Health
Organization,
"WHO Global
Action Plan
for poliovirus
containment
(GAPIII),"
2015.
[Online].
Available: http://polioeradication.org/wp-content/uploads/2016/12/GAPIII_2014.pdf. |
|
[3] World
Health
Organization,
"Guidance to
Minimize Risks
for Facilities
Collecting,
Handling or
Storing
Materials
Potentially
Infectious for
Polioviruses,"
2018.
[Online].
Available: http://polioeradication.org/wp-content/uploads/2016/07/PIM-guidance-20190122-EN.pdf. |
|
[4]
Ministry of
Health,
“Information
Session on
Poliovirus
Potentially
Infectious
Materials”,
2019.
[Online].
Available: https://www.moh.gov.sg/biosafety/newsupdate/newsdetail/Index/Information%20Session%20on%20Poliovirus%20Potentially%20Infectious%20Materials |